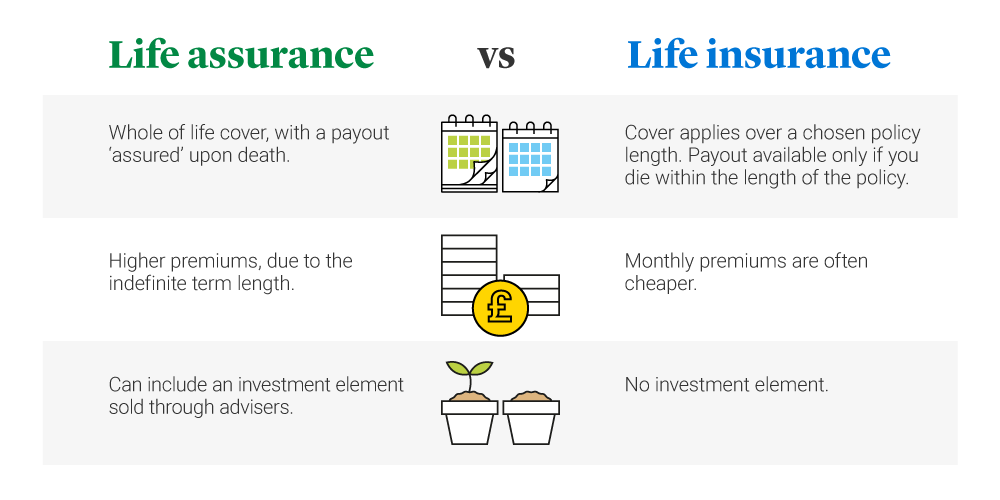
Welcome to the NSQAP Participant Portal Newborn Screening identifies conditions that can affect a childs long-term health or survival. Validation of biomarkers of CVD risk from dried blood spots in community-based research.
Cdc Newborn Screening Quality Assurance Program. NSQAP is the worlds only comprehensive program devoted to quality assurance of newborn screening using dried blood spots. Welcome to the NSQAP Participant Portal Newborn Screening identifies conditions that can affect a childs long-term health or survival. It has served as the reputable source for newborn screening quality. 404 639-3311 Public Inquiries.
 Pdf The Newborn Screening Quality Assurance Program At The Centers For Disease Control And Prevention Thirty Five Year Experience Assuring Newborn Screening Laboratory Quality From researchgate.net
Pdf The Newborn Screening Quality Assurance Program At The Centers For Disease Control And Prevention Thirty Five Year Experience Assuring Newborn Screening Laboratory Quality From researchgate.net
The Newborn Screening Quality Assurance Program NSQAP develops analytical methods to measure substances in dried blood spots DBSs and produces certified DBS quality-control and reference materials for newborn screening tests. Methodologies and study-specific serum equivalencies PDF - 169 MB. Early detection diagnosis and intervention can prevent death or disability and enable children to reach their full potential. If emailing please type 508 Accommodation PR53. These results were evaluated based on the programs. It has served as the reputable source for newborn screening quality.
Early detection diagnosis and intervention can prevent death or disability and enable children to reach their full potential.
If emailing please type 508 Accommodation PR53. Welcome to the NSQAP Participant Portal Newborn Screening identifies conditions that can affect a childs long-term health or survival. Persons with disabilities experiencing problems accessing this page should contact CDC-INFO at CDC-INFO email form. NSQAP is the worlds only comprehensive program devoted to quality assurance of newborn screening using dried blood spots.
 Source: researchgate.net
Source: researchgate.net
Newborn Screening Portal CDC Newborn screening identifies conditions that can affect a childs long-term health or survival. NSMBB Branch Priorities 1. The Newborn Screening Quality Assurance Program NSQAP at the Centers for Disease Control and Prevention CDC conducted studies to assess the individual impa. Overall Statistics Report CDC. Newborn Screening Quality Assurance Program Centers for Disease Control and Prevention CDC 4770 Buford Highway NE MSF43 Atlanta GA 30341-3724 Phone.
 Source: researchgate.net
Source: researchgate.net
Newborn Screening Quality Assurance Program. Overall Statistics Report CDC. Implement Quality Assurance Programs and provide technical support for both recent and anticipated additions to the Recommended Uniform Newborn Screening Panel RUSP 3. CDCs Division of Laboratory Sciences in the National Center for Environmental Health plays an important role in newborn screening by offering the Newborn Screening Quality Assurance Program NSQAP to local state and international laboratories and assuring newborn screening test results are as accurate as possible. Validation of biomarkers of CVD risk from dried blood spots in community-based research.

NSQAPDMTcdcgov This program is cosponsored by the Centers for Disease Control and Prevention CDC. The program provides comprehensive quality assurance resources to newborn screening labs in the US. If you have questions or access issues with forms or reporting please contact the program for help. Sustain and strengthen existing Quality Assurance Programs and services for Newborn Screening laboratories 2. Centers for Disease Control and Preventions Newborn Screening Quality Assurance Program houses a dried blood spot repository of samples containing mutations to assist newborn screening laboratories and ensure high-quality mutation detection in a high-throughput environment.
 Source: slideshare.net
Source: slideshare.net
If you have questions or access issues with forms or reporting please contact the program for help. 404 639-3311 Public Inquiries. Welcome to the NSQAP Participant Portal Newborn Screening identifies conditions that can affect a childs long-term health or survival. The Newborn Screening Contingency Plan Framework known as the Framework was developed in 2010 and the document was revised in 2015-16 in partnership. If you have questions or access issues with forms or reporting please contact the program for help.
 Source: researchgate.net
Source: researchgate.net
Newborn Screening Quality Assurance Program Centers for Disease Control and Prevention CDC 4770 Buford Highway NE MSF43 Atlanta GA 30341-3724 Phone. CDCs Newborn Screening and Molecular Biology Branch manages the Newborn Screening Quality Assurance Program NSQAP to enhance and maintain the quality and accuracy of newborn screening results. NSQAPDMTcdcgov This program is cosponsored by the Centers for Disease Control and Prevention CDC. The program provides comprehensive quality assurance resources to newborn screening labs in the US. Centers for Disease Control and Preventions Newborn Screening Quality Assurance Program houses a dried blood spot repository of samples containing mutations to assist newborn screening laboratories and ensure high-quality mutation detection in a high-throughput environment.
 Source: researchgate.net
Source: researchgate.net
Persons with disabilities experiencing problems accessing this page should contact CDC-INFO at CDC-INFO email form. Centers for Disease Control and Prevention 1600 Clifton Rd Atlanta GA 30333 USA Tel. NSQAPDMTcdcgov This program is cosponsored by the Centers for Disease Control and Prevention CDC. The program provides comprehensive quality assurance resources to newborn screening labs in the US. Httpswwwcdcgovinfo 800-232-4636 or the TTY number at 888 232-6348 and ask for a 508 Accommodation PR53.
 Source: researchgate.net
Source: researchgate.net
Newborn Screening Quality Assurance Program. Welcome to the NSQAP Participant Portal Newborn Screening identifies conditions that can affect a childs long-term health or survival. Centers for Disease Control and Prevention 1600 Clifton Rd Atlanta GA 30333 USA Tel. Newborn Screening Portal CDC Newborn screening identifies conditions that can affect a childs long-term health or survival. NSQAP is the worlds only comprehensive program devoted to quality assurance of newborn screening using dried blood spots.
 Source: slideplayer.com
Source: slideplayer.com
Centers for Disease Control and Preventions Newborn Screening Quality Assurance Program houses a dried blood spot repository of samples containing mutations to assist newborn screening laboratories and ensure high-quality mutation detection in a high-throughput environment. It has served as the reputable source for newborn screening quality. 404 639-3311 Public Inquiries. Methodologies and study-specific serum equivalencies PDF - 169 MB. Sustain and strengthen existing Quality Assurance Programs and services for Newborn Screening laboratories 2.
 Source: researchgate.net
Source: researchgate.net
Centers for Disease Control and Prevention 1600 Clifton Rd Atlanta GA 30333 USA Tel. The Newborn Screening Quality Assurance Program NSQAP develops analytical methods to measure substances in dried blood spots DBSs and produces certified DBS quality-control and reference materials for newborn screening tests. Newborn Screening Portal CDC Newborn screening identifies conditions that can affect a childs long-term health or survival. Newborn Screening Quality Assurance Program Centers for Disease Control and Prevention CDC 4770 Buford Highway NE MSF43 Atlanta GA 30341-3724 Phone. Newborn Screening Quality Assurance Program CF DNA Proficiency Testing PT Program Each participating laboratory received 5 blind-coded profi-ciency testing specimens 4 times a year and laboratories reported both the genotyping results and clinical assessments to CDC.

Sustain and strengthen existing Quality Assurance Programs and services for Newborn Screening laboratories 2. Sustain and strengthen existing Quality Assurance Programs and services for Newborn Screening laboratories 2. The Newborn Screening Contingency Plan Framework known as the Framework was developed in 2010 and the document was revised in 2015-16 in partnership. 4 Amino Acids Specimen 41651 Arg Cit Leu Met Phe SUAC Tyr Val N 237 260 293 271 378 115 295 262 Outliers 8 11 5 12 24 5 9 3 Mean 1142 1697 13575 1501 5199 054 5098 12477. Httpswwwcdcgovinfo 800-232-4636 or the TTY number at 888 232-6348 and ask for a 508 Accommodation PR53.
 Source: researchgate.net
Source: researchgate.net
NSQAPDMTcdcgov This program is cosponsored by the Centers for Disease Control and Prevention CDC. The program provides comprehensive quality assurance resources to newborn screening labs in the US. 404 639-3311 Public Inquiries. 4 Amino Acids Specimen 41651 Arg Cit Leu Met Phe SUAC Tyr Val N 237 260 293 271 378 115 295 262 Outliers 8 11 5 12 24 5 9 3 Mean 1142 1697 13575 1501 5199 054 5098 12477. The Newborn Screening Contingency Plan Framework known as the Framework was developed in 2010 and the document was revised in 2015-16 in partnership.
 Source: slideshare.net
Source: slideshare.net
NSQAPDMTcdcgov This program is cosponsored by the Centers for Disease Control and Prevention CDC. CDCs Division of Laboratory Sciences in the National Center for Environmental Health plays an important role in newborn screening by offering the Newborn Screening Quality Assurance Program NSQAP to local state and international laboratories and assuring newborn screening test results are as accurate as possible. Newborn Screening Quality Assurance Program Centers for Disease Control and Prevention CDC 4770 Buford Highway NE MSF43 Atlanta GA 30341-3724 Phone. Newborn Screening Portal CDC Newborn screening identifies conditions that can affect a childs long-term health or survival. Implement Quality Assurance Programs and provide technical support for both recent and anticipated additions to the Recommended Uniform Newborn Screening Panel RUSP 3.
 Source: slideshare.net
Source: slideshare.net
The Newborn Screening Quality Assurance Program NSQAP at the Centers for Disease Control and Prevention CDC conducted studies to assess the individual impa. Newborn Screening Quality Assurance Program. The Newborn Screening Quality Assurance Program NSQAP at the Centers for Disease Control and Prevention CDC conducted studies to assess the individual impa. These results were evaluated based on the programs. NSQAPDMTcdcgov This program is cosponsored by the Centers for Disease Control and Prevention CDC.
 Source: researchgate.net
Source: researchgate.net
Centers for Disease Control and Prevention 1600 Clifton Rd Atlanta GA 30333 USA Tel. Methodologies and study-specific serum equivalencies PDF - 169 MB. If emailing please type 508 Accommodation PR53. Centers for Disease Control and Preventions Newborn Screening Quality Assurance Program houses a dried blood spot repository of samples containing mutations to assist newborn screening laboratories and ensure high-quality mutation detection in a high-throughput environment. 404 639-3311 Public Inquiries.

NSQAPDMTcdcgov This program is cosponsored by the Centers for Disease Control and Prevention CDC. 4 Amino Acids Specimen 41651 Arg Cit Leu Met Phe SUAC Tyr Val N 237 260 293 271 378 115 295 262 Outliers 8 11 5 12 24 5 9 3 Mean 1142 1697 13575 1501 5199 054 5098 12477. NSMBB Branch Priorities 1. Validation of biomarkers of CVD risk from dried blood spots in community-based research. Implement Quality Assurance Programs and provide technical support for both recent and anticipated additions to the Recommended Uniform Newborn Screening Panel RUSP 3.
 Source: researchgate.net
Source: researchgate.net
Welcome to the NSQAP Participant Portal Newborn Screening identifies conditions that can affect a childs long-term health or survival. Methodologies and study-specific serum equivalencies PDF - 169 MB. Centers for Disease Control and Prevention 1600 Clifton Rd Atlanta GA 30333 USA Tel. The program provides comprehensive quality assurance resources to newborn screening labs in the US. CDCs Division of Laboratory Sciences in the National Center for Environmental Health plays an important role in newborn screening by offering the Newborn Screening Quality Assurance Program NSQAP to local state and international laboratories and assuring newborn screening test results are as accurate as possible.
 Source: researchgate.net
Source: researchgate.net
It has served as the reputable source for newborn screening quality. Centers for Disease Control and Preventions Newborn Screening Quality Assurance Program houses a dried blood spot repository of samples containing mutations to assist newborn screening laboratories and ensure high-quality mutation detection in a high-throughput environment. Newborn Screening Quality Assurance Program. Implement Quality Assurance Programs and provide technical support for both recent and anticipated additions to the Recommended Uniform Newborn Screening Panel RUSP 3. Persons with disabilities experiencing problems accessing this page should contact CDC-INFO at CDC-INFO email form.
 Source: researchgate.net
Source: researchgate.net
4 Amino Acids Specimen 41651 Arg Cit Leu Met Phe SUAC Tyr Val N 237 260 293 271 378 115 295 262 Outliers 8 11 5 12 24 5 9 3 Mean 1142 1697 13575 1501 5199 054 5098 12477. Newborn Screening Quality Assurance Program. 404 639-3311 Public Inquiries. CDCs Division of Laboratory Sciences in the National Center for Environmental Health plays an important role in newborn screening by offering the Newborn Screening Quality Assurance Program NSQAP to local state and international laboratories and assuring newborn screening test results are as accurate as possible. Newborn Screening Quality Assurance Program Centers for Disease Control and Prevention CDC 4770 Buford Highway NE MSF43 Atlanta GA 30341-3724 Phone.